When physicians around the world count on your products to diagnose and treat countless patients, you need to make sure those products are safe and as affordable as possible.
That’s why Boston Scientific Heredia evaluates its operations regularly, to maintain process efficiency and contribute to affordable healthcare by reducing costs. Boston Scientific has developed, manufactured, and marketed medical technology for over 30 years, and serves a broad range of medical specialties.
At its Heredia, Costa Rica facility, packaging engineer Daniel Calvo Camacho led an effort to streamline packaging for guidewires—which are used during procedures such as catheter placement or endoscopic diagnoses—with the introduction of a new, smaller plastic pouch.
Engineers at the facility used Minitab Statistical Software to help assess and validate their packaging process, which allowed Boston Scientific to proceed with the cost-saving redesign of its pouches with total confidence.
The Challenge
Using smaller and different packaging materials for their Amplatz Super Stiff Guidewires would substantially reduce Boston Scientific’s material costs, but the company needed to make sure the new pouches would work with their sealing process, which creates a barrier that keeps the guidewires sterile until they’re ready for use.

A project team at Boston Scientific’s Costa Rica facility used Minitab Statistical Software to analyze data that would validate the pouch-sealing process for packaging the guidewires used for medical procedures. Above, the guidewires are shown loaded in a pouch, ready for sealing.
In a cleanroom, operators load the guidewires into pouches, then place the pouches upside-down on the sealer-conveyer. The open edge of each pouch passes between front and rear bands that apply heat and pressure, creating a peelable seal.
The packaging engineers needed to make sure that the strength of the seal on the smaller pouches still met or exceeded standards. They evaluated the process and identified several input variables, which included the temperatures of the sealing system’s front and rear heating bands and the speed of the system.
Now the team needed to assess each of these variables and determine how they affected the quality of the pouch seal. Which inputs were most important? And where should the operators set the temperatures and speed of the system to ensure each pouch had a strong seal?
How Minitab Helped
That’s where the statistical method called Design of Experiments comes in. A designed experiment lets you simultaneously change and assess more than one input variable at a time, then uses statistical analysis to get meaningful results about all input variables of interest. You can quickly determine which factors matter, and then adjust the process to get the desired results.
Minitab Statistical Software can be used to easily create and analyze many kinds of designed experiments. Calvo Camacho used it to create a two-level, full factorial designed experiment, which would enable the team to evaluate all of the input variables and the two-way interactions between them.
“After evaluating the preliminary process parameters to create the DOE, I chose the different factors and levels of those factors that would aid the team in executing our experiments,” says Calvo Camacho. The team then set about collecting the experimental data. Minitab’s DOE tool created a list of runs, each specifying different combinations of the two levels of each factor. Operators produced a sample seal for each experimental condition, then Calvo Camacho and his team measured the strength of each seal. After completing all experimental runs, he used Minitab to analyze the results.
The analysis identified front and rear temperatures as significant factors, as well as the two-way interaction between them. Speed was not a significant factor for seal strength, which allowed the team to remove it from the model. Traditional one factor at a time (OFAT) experimentation could have easily missed the significant interaction leading to process control issues in the long run.
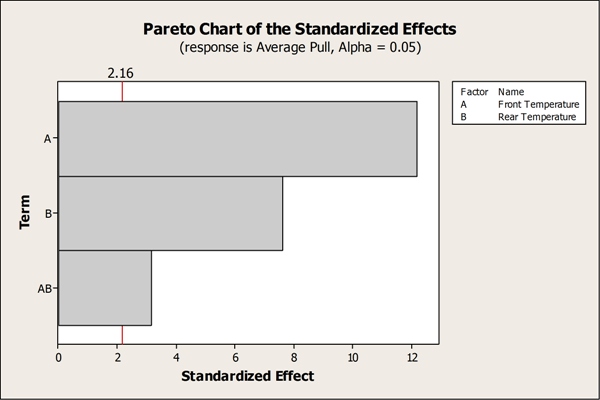
The Pareto Chart above identifies the factors that significantly affect seal strength: front temperature, rear temperature, and their respective two-way interaction.
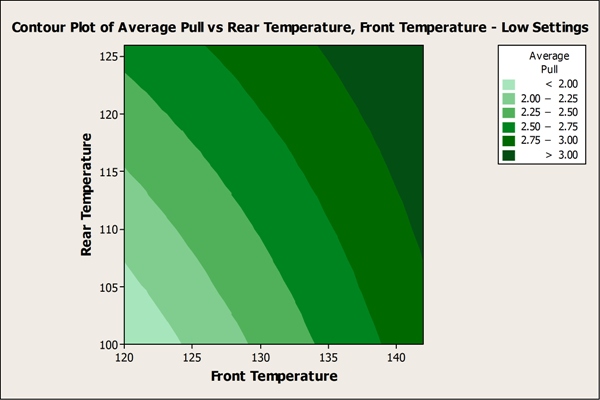
The team also used Minitab to create contour plots that reveal the temperature ranges under which seal strength met or exceeded specifications.
By focusing on the critical factors identified in the designed experiment, the team devised optimal process settings to ensure the new pouches had strong seals. Now they turned to Minitab to verify the effectiveness of the new process settings with a tool called capability analysis, which demonstrates whether or not a process meets specifications and can produce good parts. They sealed the pouches using the optimal process settings they had identified, keeping in mind that the higher the temperatures, the stronger the seal. They then measured the seal strength of these pouches, and performed a capability analysis to assess the measurements relative to the lower specification limit. Their capability analysis showed that the proposed temperature settings met, and even exceeded, the minimum seal strength requirements.
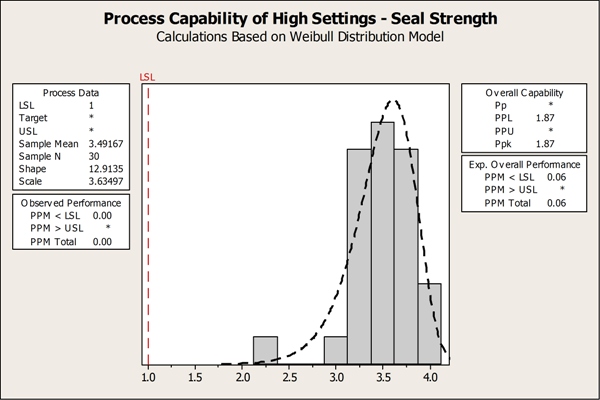
The team performed process capability analysis in Minitab, which helped them to prove that the proposed temperature settings would allow them to achieve end production goals.
Through experiments and the data analysis they performed using Minitab Statistical Software, the team was able to demonstrate that their packaging system functioned in a highly efficient manner, and would continue to do so with the new pouch.
Results
Now that careful data analysis has shown the effectiveness of the sealing system, the facility in Costa Rica can begin using the new plastic pouch, which will result in significant savings. “We evaluate our processes annually in order to always improve quality and service, but we are also aggressive in pursuing opportunities that will reduce costs,” Calvo Camacho says.
Adopting the new pouch supports Boston Scientific’s broader efforts to streamline packaging across the corporation, and the project played a major role in the company’s combined savings of more than $330,000 to date. What’s more, the team’s process validation confirms that the changes—and savings—can be sustained. That’s good news for the company, and ultimately for the physicians and patients who rely on their products.
“At the end of the day,” Calvo Camacho notes, “the more money we save, the more additional savings we can pass on to the people we serve.”

Organization
Boston Scientific
Overview
- One of the world’s largest developers, manufacturers, and marketers of medical devices
- Products used to treat a wide range of medical conditions, including heart, digestive, pulmonary, vascular, urological, women’s health, and chronic pain
- Employs approximately 23,000 worldwide
Challenge
Evaluate the pouch-sealing process in order to define optimum process conditions and reduce material costs at the Heredia, Costa Rica site.
Products Used
Minitab® Statistical Software
Results
- Used data analysis to validate the pouch sealing process and identify key inputs
- Introduced a new pouch that increased material cost savings
- Streamlined packaging for guidewires across several areas of the corporation



